Brand/nama lain
Simponi/ testing
Cara Kerja
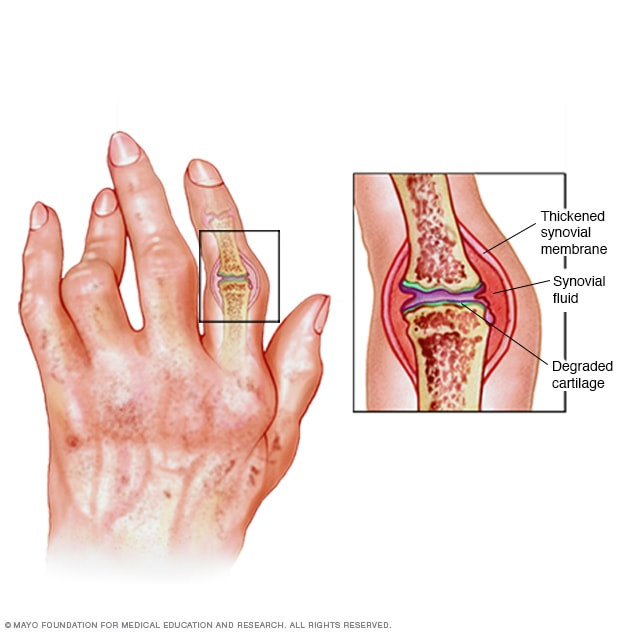
Golimumab merupakan penghambat faktor nekrosis tumor (TNF inhibitor) yang digunakan untuk meredakan inflamasi yang terjadi pada kondisi medis tertentu. Obat ini bekerja dengan cara menghambat efek TNF, yaitu protein yang diproduksi tubuh saat terjadi peradangan. Tujuan dari obat ini adalah untuk memblokade efek protein TNF yang berlebihan yang dihasilkan oleh sistem imun saat inflamasi, sehingga mampu mengurangi kerusakan pada sel dan jaringan. Dengan dihambatnya efek TNF, peradangan dapat mereda, sehingga kerusakan sel dan jaringan dapat diperlambat atau dicegah.
Indikasi
Golimumab adalah suatu obat yang digunakan untuk meredakan gejala beberapa penyakit autoimun, yaitu suatu kondisi ketika sistem imun menyerang bagian tubuh yang sehat sehingga menimbulkan rasa nyeri, pembengkakan, dan kerusakan, antara lain:
- Rheumatoid arthritis, yaitu kondisi ketika sistem imun tubuh menyerang sendi dan menyebabkan rasa nyeri, pembengkakan, dan gangguan fungsi sendi
- Ankylosing spondylitis, yaitu kondisi ketika sistem imun tubuh menyerang sendi pada tulang belakang dan area lain yang menyebabkan rasa nyeri dan kerusakan sendi
- Psoriatic arthritis, yaitu kondisi yang menyebabkan rasa nyeri sendi, pembengkakan, dan muncul sisik pada kulit
- Kolitis ulseratif, yaitu kondisi yang menyebabkan pembengkakan dan luka pada dinding saluran pencernaan
- Polyarticular juvenile idiopathic arthritis (pcJIA), yaitu kondisi inflamasi kronik pada anak, terjadi pada usia < 16 tahun pada setidaknya 1 sendi selama kurang lebih 6 bulan tanpa penyebab lain.
Obat ini dapat digunakan sebagai terapi tunggal atau dikombinasikan dengan methotrexate.
Kontraindikasi
Obat ini tidak boleh digunakan oleh orang yang memiliki beberapa kondisi di bawah ini:
- Hipersensitivitas (reaksi sistem imun yang berlebihan) terhadap golimumab
- Infeksi berat (seperti tuberkulosis, sepsis, infeksi oportunistik)
- Gangguan fungsi hati sedang hingga berat
- Gagal jantung berat (klasifikasi NYHA III/IV)
- Ibu hamil dan menyusui
Efek Samping
Beberapa efek samping yang dapat timbul akibat penggunaan Golimumab, seperti:
- Rasa nyeri atau gatal pada area bekas suntikan
- Gejala mirip flu atau pilek
- Ruam kulit
- Pusing dan tekanan darah tinggi
Efek samping yang lebih serius perlu ditangani oleh dokter segera, antara lain:
- Kelelahan ekstrim
- Nyeri sendi
- Nyeri dada
- Sesak napas
- Pembengkakan pada kaki
- Gangguan penglihatan
- Tangan atau kaki melemah
- Bentol-bentol merah pada kulit dan berisi nanah
- Kulit pucat
- Pembengkakan mata, wajah, bibir, lidah, mulut, atau tenggorokan
Sediaan
Golimumab tersedia dalam sediaan injeksi berbentuk suntikan siap pakai yang disuntikkan ke lapisan di bawah kulit atau pembuluh darah. Sebagai terapi untuk rheumatoid arthritis, psoriatic arthritis, atau ankylosing spondylitis, golimumab dapat disuntikkan ke bawah lapisan kulit maupun ke dalam pembuluh darah. Simponi (merk dagang Golimumab) tersedia dalam bentuk injeksi prefilled syringe 50 mg/0,5 mL, 100 mg/mL, intravena 50 mg/4mL, serta autoinjector Simponi SmartJect 50 mg/0,5 mL dan 100 mg/mL.
Dosis
Golimumab akan disuntikkan ke bawah kulit (subkutan/SC) atau melalui pembuluh darah (intravena/IV) oleh dokter atau petugas medis di bawah pengawasan dokter. Penyuntikan golimumab ke lapisan bawah kulit untuk pertama kali biasanya dilakukan oleh dokter. Setelah itu, dokter akan memperbolehkan Anda (sebagai pasien) atau keluarga untuk menyuntikkan obat secara mandiri.
Berikut ini adalah dosis umum penggunaan golimumab berdasarkan kondisi yang ditangani:
Rheumatoid arthritis
- Suntikan IV pada orang dewasa:
Dosis awal 2 mg/kgBB yang diberikan melalui infus selama 30 menit dikombinasikan dengan methotrexate. Dosis kedua diberikan 4 minggu kemudian dan selanjutnya diberikan setiap 8 minggu.
- Suntikan SC pada orang dewasa:
Dosis 50 mg satu bulan sekali pada tanggal yang sama dikombinasikan dengan methotrexate. Untuk pasien dengan berat badan >100 kg, dosis yang dapat diberikan adalah 100 mg.
Ankylosing spondylitis, Non-radiographic axial spondyloarthritis, Psoriatic arthritis
- Suntikan SC pada orang dewasa:
Dosis 50 mg satu bulan sekali pada tanggal yang sama, dapat digunakan sebagai obat tunggal atau dikombinasikan dengan obat antirheumatik (disease-modifying antirheumatic drugs) non-biologis lainnya. Untuk pasien dengan berat badan >100 kg, dosis yang dapat diberikan adalah 100 mg.
Juvenile idiopathic arthritis
- Suntikan SC pada anak-anak dengan berat badan ≥40 kg:
Dosis 50 mg satu bulan sekali pada tanggal yang sama.
Kolitis ulseratif
- Suntikan SC pada orang dewasa:
Dosis awal 200 mg, diikuti 100 mg setelah 2 minggu. Kemudian, 50 mg untuk pasien dengan berat badan <80 kg, atau 100 mg untuk pasien dengan berat badan ≥80 kg setiap 4 minggu.
Keamanan
- Kehamilan:
Golimumab dalam sediaan injeksi termasuk dalam FDA Kategori C yang mengindikasikan bahwa obat berisiko menyebabkan gangguan kehamilan. Oleh karena itu, obat kategori C hanya dianjurkan jika manfaat yang diperoleh ibu maupun janin lebih besar daripada risiko yang ditimbulkannya.
- Menyusui:
Belum diketahui apakah golimumab dapat terserap ke dalam ASI atau tidak. Bila Anda sedang menyusui, jangan menggunakan obat ini tanpa berkonsultasi dulu dengan dokter.
Interaksi Obat
Golimumab tidak boleh digunakan bersamaan dengan sediaan biologis untuk terapi reumathoid artritis lain, seperti abatacept dan anakinra, serta bersamaan dengan pemberian vaksin hidup karena dapat meningkatkan risiko terkena infeksi serius.
Berikut adalah efek interaksi obat yang dapat terjadi jika golimumab digunakan bersamaan dengan obat lainnya:
- Peningkatan kadar golimumab dalam darah jika digunakan dengan methotrexate
- Peningkatan kadar dan efek terapi dari ciclosporin, teofilin, atau warfarin
- Peningkatan risiko terjadinya neutropenia dan infeksi serius jika digunakan dengan anakinra
- Peningkatan risiko terjadinya infeksi serius jika digunakan dengan abatacept, rituximab, atau tocilizumab
Mau tahu informasi seputar obat-obatan lainnya? Cek di sini, ya!
- dr. Alvidiani Agustina Damanik
U.S. National Library of Medicine. National Institute of Health MedlinePlus. Golimumab Injection. Retrieved 22 July 2024, from https://medlineplus.gov/druginfo/meds/a610010.html
MIMS Indonesia. Golimumab. Retrieved 22 July 2024, from https://www.mims.com/indonesia/drug/info/golimumab?mtype=generic
Drugbank. Golimumab. Retrieved 23 July, 2024, from https://go.drugbank.com/drugs/DB06674
New York WebMD. Golimumab Solution. Retrieved 23 July 2024, from: https://www.webmd.com/drugs/2/drug-164761/golimumab-intravenous/details
Electronic Medicines Compendium. Simponi 50 Mg Solution for Injection in Pre-Filled Syringe. Retrieved 23 July 2024, from: https://www.medicines.org.uk/emc/product/5707/smpc#gref
Mayo Clinic Drugs and Supplements. Golimumab (Intravenous Route, Subcutaneous Route). Retrieved 22 July 2024, from: https://www.mayoclinic.org/drugs-supplements/golimumab-intravenous-route-subcutaneous-route/description/drg-20073012#:~:text=Descriptions,some%20areas%20of%20the%20body.
Medscape Drugs & Diseases. Golimumab (Rx). Retrieved 23 July 2024, from https://reference.medscape.com/drug/simponi-golimumab-999102

/669f34947bb6a.jpg)